Know the CR Industry
What Is Clinical Research?
I hope many of you already know “What Clinical Research exactly is?”. Still, lets take a quick overview of it. So, here is the definition you may be looking for. To be precise, “It is a systematic study of new drug in human subject to generate data for discovering the clinical, pharmacological, and adverse effects with the objective of determining safety and efficacy of the new drug.”
Enquire Here For Our Courses

Firstly, you must know that, Clinical Research is an important field as it is making positive changes in the healthcare industry. Hence, clinical research assists in the new drug inventions, new uses of existing drug and new medical device invention. Also, it helps in making a new drug delivery system as well as in new surgical procedures.
Furthermore, the field of clinical research is vast and has several sub-domains under it. Again, some of these are Clinical Trial Management, Clinical Data Management, Pharmacovigilance, etc.
Enroll with Canvass Clinical Research Institute
Working in this industry requires a detailed knowledge. For this purpose, we conduct diploma and degree courses in the domain of clinical research for the students from life science backgrounds. In that way, we help the students to grab jobs in the clinical research industry.
Domains of Clinical Research
In brief, this growing industry has several sub-domains. Each domain plays a fundamental role in conducting a clinical trial.
Clinical Trial Management
Initially, in this domain, clinical trial are conducted in multispecialty hospitals on particular molecule of sponsor. Hence, the overall process is carried out under the observation of Principle Investigator.
Clinical Data Management & Medical Coding
Subsequently, Clinical Data Management ensures collection, integration and availability of data from clinical trials. Whereas, Medical coders transform the clinical trial information into universal medical alphanumeric codes.
Pharmacovigilance & Medical Writing
Likewise, pharmacovigilance means watch on drug. To be said, it is the pharmacological science relating to the collection, detection, assessment, monitoring, and prevention of adverse effects with pharmaceutical products.
Regulatory Affair
Besides, RA also called as Government Affairs, is a profession within regulated industries, such as pharmaceuticals, medical devices, etc. CDSCO (Central Drug Standard)
e Trial Master File
Again, an electronic master file or eTMF is a Trial Master File in electronic or digital format. To rephrase it, it is not only a way of digitally capturing essential documents form a clinical trial but also managing, sharing and storing them.
Biostatics & SAS
Finally, SAS Clinicals is a Statistical Analysis Software developed for data management. Moreover, it brings automation to the data integration process.
Phases of the Clinical Trials
Lastly, there are total four phases involved in any clinical trial. In addition, the stakeholders / players in clinical research play an important role in each phase of the trials.
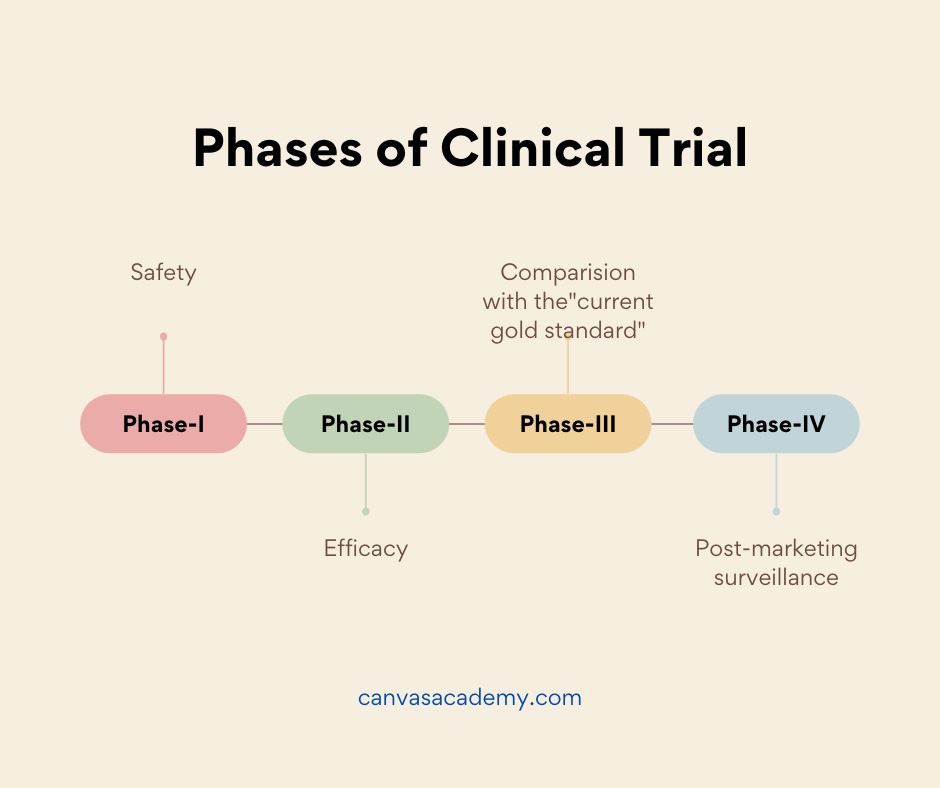
To conclude, I hope that you may have now understood about the clinical research domain in detail. What’s more, is Canvass Clinical Research Institute offers a variety of clinical research courses. So that, students could enroll in them and pursue a successful career in it. Click here to contact us.

Pingback: Scope of Clinical Research In India -